Details for: DICLOFENAC
Company: PHARMA STULLN INC.
| DIN | DIN name | Active Ingredient(s) | Strength | Dosage Form | Route of Administration |
|---|---|---|---|---|---|
| 02475065 | DICLOFENAC | DICLOFENAC SODIUM | 0.1 % / W/V | SOLUTION | OPHTHALMIC |
Consumer Information
This information was provided by the drug’s manufacturer when this drug product was approved for sale in Canada. It is designed for consumers and care givers. It is a summary of information about the drug and will not tell you everything about the drug. Contact your doctor or pharmacist if you have any questions about the drug.
What the medication is used for
Your doctor has prescribed DICLOFENAC (diclofenac sodium) solution, 0.1% w/v for you which is a non-steroidal anti-inflammatory drug (NSAID), used to treat eye inflammation after cataract surgery and eye inflammation after a non-penetrating eye injury.
What it does
DICLOFENAC eye drops reduce pain and inflammation by reducing the production of certain substances called prostaglandins.
When it should not be used
DO NOT USE DICLOFENAC eye drops
if you are allergic to diclofenac sodium, any other
ingredient in the formulation (see What the non-medicinal ingredients are), or other medications of
the NSAID group, such as acetylsalicylic acid,
diflunisal, ibuprofen, flurbiprofen, ketoprofen,
indomethacin, mefenamic acid, piroxicam, sulindac,
or tiaprofenic acid.
DICLOFENAC is not for use in children under 18
years of age.
What the medicinal ingredient is
Diclofenac sodium, 0.1% w/v
What the non-medicinal ingredients are
Boric Acid, Trometamol, Macrogolglycerol Ricinoleate, Sodium Hydroxide, Purified Water.
What dosage form it comes in
0.1% (diclofenac sodium) w/v ophthalmic solution is available in plastic unit dose container of 0.3 mL.
Warnings and precautions
BEFORE taking DICLOFENAC, tell your doctor or pharmacist if you:
- are pregnant or intend to become pregnant while taking this medication;
- are breast feeding or planning to breastfeed;
- are taking a topical corticosteroid. Taking DICLOFENAC and a corticosteroid at the same time may slow wound healing;
- have (had) complicated eye surgery, pre-existing corneal problems, diabetes, problems with your eye surface (such as dry eye), rheumatoid arthritis or multiple eye surgeries. You may be a higher risk for developing serious eye side effects.
- Taking DICLOFENAC more than 24 hours before eye surgery or for more than 14 days after surgery may also increase your risk for developing serious eye side effects;
- have any other medical problem(s);
- wear soft contact lenses. DO NOT administer DICLOFENAC while wearing soft contact lenses. Remove lenses before application and reinsert no earlier than 15 minutes after use.
WHILE taking DICLOFENAC:
- check with your doctor if you are not getting any relief or if any problems develop, such as an eye infection or bleeding problems;
- report any reactions to your doctor. This is very important because it will help in the early detection and prevention of problems;
- If you experience any vision problems, in particular blurring of vision, DO NOT drive or operate any machinery.
STOP using DICLOFENAC and talk to your
doctor if you experience any serious problems with
your eye(s).
Your regular medical check-ups, including
monitoring of eye pressure, are essential.
Interactions with this medication
BEFORE taking DICLOFENAC, tell your doctor or pharmacist if you are taking any other drug (either prescription or non-prescription) such as:
- corticosteroids
- medications that prolong bleeding time
- antibiotics
Proper use of this medication
DICLOFENAC is for topical use only.
Usual adult dose:
Cataract surgery
Before surgery: Apply 1 drop into the affected eye(s)
up to 5 times during the 3 hours before your
scheduled surgery.
After surgery: Apply 1 drop into the affected eye(s)
15, 30 and 45 minutes following surgery. Then apply
1 drop 3 to 5 times per day for up to 4 weeks.
Inflammation from non-penetrating wounds Apply
1 drop into the affected eye(s) 4 to 5 times per day as
directed by your physician.
How to use this medication:
Open the drops container just before you want to use
it. After using the drops, throw away what is left. This
is because the drops cannot be kept free of bacteria
after being opened.
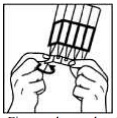
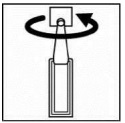
- First wash your hands and then break off a single dose container from the strip. Then twist open the top of the single dose container as shown. Each single dose container contains enough solution for both eyes. If you wear contact lenses, remove them before using your eye drops.
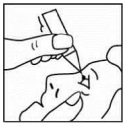
- Pull down your lower eyelid with a clean finger until there is a ‘pocket’ between the eyelid and your eye. This is where the drop will go. Bring the single dose container close to the eye. Do this in front of the mirror if it helps.
- Close your eyelid and gently press the inner corner of your eye with your forefinger for 2 minutes.
- Wipe any excess liquid from your face with a tissue. If you must use more than 1 drop in the same eye, wait at least 5 minutes before applying the next drop. Eye ointments should be applied last.
- Wash your hands to remove any medication. Discard unused portion.

Overdose
Overdosage will not usually cause sudden problems. If DICLOFENAC is accidentally ingested, fluids should b e taken to dilute the medication.
Missed Dose
If a dose of this medication has been missed, it should be taken as soon as possible. However, if it is almost time for the next dose, skip the missed dose and go back to the regular dosing schedule. DO NOT double doses.
Side effects and what to do about them
Occasionally you may experience a mild to moderate
burning sensation when DICLOFENAC is instilled in
the eye. This symptom usually disappears rapidly, but
if it or any other side effects persist, check with your
doctor.
Less frequently observed eye side effects are allergic
reaction, itchy eye(s), reddening of eye and blurred
vision immediately after instillation of the eye drops,
eye pain, eye surface inflammation with surface
damage, sensitivity to light, abnormal vision, eye
allergy, eye swelling, clouding of the eye surface,
eyelid swelling, eye irritation, eye discharge, eyelid
reddening, swelling or rash, eye crusting, eye
discomfort, slower healing and a stainable cornea.
Uncommon side effects in the rest of the body are bad
taste, feeling of pressure, abdominal pain, feeling
weak, chills, dizziness, swelling of the face, fever,
headache, problems sleeping, nausea, pain, nose
irritation, a viral infection, hives, rash, eczema, skin
redness, cough, allergic reaction and vomiting.
If you are using DICLOFENAC after cataract
surgery, you may feel increased eye pressure
(intraocular pressure).
If you are using DICLOFENAC after refractive
surgery, you may notice tearing.
If you use corticosteroids, have infections or have
rheumatoid arthritis, you may develop ulcers,
thinning or inflammation of your cornea, which may
cause loss of vision.
Report any reactions to your doctor. This is very
important because it will help in the early detection
and prevention of problems.
What Diclofenac UD looks like and contents of the
pack
Unit dose containers made of LDPE with:
10 x 0.3 ml
20 x 0.3 ml
50 x 0.3 ml
| Symptom / effect | Talk with your doctor, nurse, or pharmacist only if severe | Talk with your doctor, nurse, or pharmacist in all cases | Stop taking drug and get immediate medical help |
|---|---|---|---|
| Uncommon | |||
| Ulcer, thinning or swelling of your cornea | ✔ | ||
| Outer layer defects of your cornea | ✔ | ||
| Shortness of breath | ✔ | ||
| Increase in signs and symptoms of asthma | ✔ | ||
| Severe allergic reaction | ✔ | ||
| Unknown | |||
| Tiny tears (perforations) In your cornea | ✔ | ||
This is not a complete list of side effects. For any unexpected effects while taking DICLOFENAC, contact your doctor or pharmacist.
How to store
Store the unique dose container at room temperature
between 15°and 30 °C. Protect from light.
Keep out of the reach and sight of children.
Reporting side effects
You can report any suspected adverse reactions associated with the use of health products to the Canada Vigilance Program by one of the following three ways:
- Report online at www.healthcanada.gc.ca/medeffect
- Call toll-free at 1-866-234-2345
- Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-866-678-6789, or
- Mail to:
Canada Vigilance Program
Health Canada
Postal Locator 0701E
Ottawa ON K1A 0K9
Postage paid labels, Canada Vigilance Reporting Form and the adverse reaction reporting guidelines are available on the MedEffectTM Canada Web site at www.healthcanada.gc.ca/medeffect.
NOTE: Should you require information related to the management of side effects, contact your health professional. The Canada Vigilance Program does not provide medical advice.
More information
For more information, please contact your doctor,
pharmacist or other healthcare professional.
This leaflet plus the full product monograph,
prepared for health professionals, can be found in the
Health Canada website or by contacting.
Pharma Stulln Inc.
6500 TransCanada Highway, Suite 400
Pointe Claire, Quebec
H9R 0A5
E-mail: info@pharmastulln.ca
Last revised: June 19, 2018