Details for: TEVA-COMBO STERINEBS
Company: TEVA CANADA LIMITED
| DIN | DIN name | Active Ingredient(s) | Strength | Dosage Form | Route of Administration |
|---|---|---|---|---|---|
| 02272695 | TEVA-COMBO STERINEBS | SALBUTAMOL (SALBUTAMOL SULFATE); IPRATROPIUM BROMIDE (IPRATROPIUM BROMIDE MONOHYDRATE) | 2.5 MG / 2.5 ML; 0.5 MG / 2.5 ML | SOLUTION | INHALATION |
Consumer Information
This information was provided by the drug’s manufacturer when this drug product was approved for sale in Canada. It is designed for consumers and care givers. It is a summary of information about the drug and will not tell you everything about the drug. Contact your doctor or pharmacist if you have any questions about the drug.
What the medication is used for
TEVA-COMBO Sterinebs® is used to treat the wheezing or shortness of breath caused by COPD (chronic obstructive pulmonary disease which includes chronic bronchitis and emphysema).
What it does
TEVA-COMBO Sterinebs®
is a combination of two drugs
that are bronchodilators: ipratropium bromide (an
anticholinergic) and salbutamol (a beta-agonists).
TEVA-COMBO Sterinebs®
works by relaxing the muscle
surrounding the bronchi (airways in the lungs) and
therefore helps to ease breathing problems.
You may already be familiar with one or both of
these bronchodilators, since they are also
available separately, with a prescription as TevaIpratropium
(Ipratropium bromide) and TevaSalbutamol
(salbutamol).
When it should not be used
Do not take TEVA-COMBO Sterinebs®
- if you are allergic to ipratropium bromide or other drugs which are anticholinergic (contain atropine or its derivatives), salbutamol sulphate, or to any component of TEVA-COMBO Sterinebs® (see “What the nonmedicinal ingredients are”);
- if you have a fast or irregular heart beat or have a thickened heart muscle due to various conditions;
- if you are under 18 years of age.
What the medicinal ingredient is
Ipratropium bromide monohydrate and salbutamol sulphate.
What the non-medicinal ingredients are
Sodium chloride and hydrochloric acid.
What dosage form it comes in
TEVA-COMBO Sterinebs® (Salbutamol Sulphate and Ipratropium Bromide inhalation solution) is supplied in plastic single dose units in strips of 5 with a foil overwrap, each containing 0.5 mg ipratropium bromide (as monohydrate) and 2.5 mg salbutamol (as salbutamol sulphate) in a 2.5 mL isotonic preservative-free solution for inhalation by ventilator or compressor-driven nebulizer. There are 4 strips per carton for a carton size of 20 unit dose vials
Warnings and precautions
The solution is intended for inhalation only. Do
not inject or drink.
Do not let the nebulized mist get into your eyes
as this may cause blindness known as acute angle
glaucoma. This may present as eye pain or
discomfort, blurred vision, visual halos or
coloured images in association with red eyes. If
any combination of these symptoms occurs, seek
immediate medical attention. Patients with
glaucoma should use swimming goggles or a
nebulizer with a mouthpiece to prevent nebulized
solution getting into the eyes.
BEFORE you use TEVA-COMBO Sterinebs®
talk to your
doctor or pharmacist if you:
- are pregnant or intend to become pregnant;
- are breast feeding;
- are having treatment for a thyroid or adrenal gland condition;
- are having treatment for high blood pressure, angina or a heart problem;
- have diabetes;
- have low levels of potassium in your blood
(hypokalemia), especially if you are taking:
- drugs known as xanthine derivatives (such as theophylline)
- steroids to treat asthma
- water pills (diuretics)
- have eye problems, such as glaucoma, or eye pain;
- are taking any other medications including eye drops or any medications you can buy without a prescription;
- have difficulty in urination;
- have enlarged prostate;
- have any allergies or reactions to foods or drugs;
- have a history of convulsions (uncontrolled shaking or seizures);
- have liver or kidney disease.
- you require more than one dose to relieve your breathing problems;
- your shortness of breath becomes worse;
- you don’t get the same benefit from your medicine as you did before;
- you have breathing difficulties and chest pain;
- you experience difficulty with urination.
The use of TEVA-COMBO Sterinebs® may test positive for performance enhancement (doping) in athletic competition.
Interactions with this medication
As with most medicines, interactions with other
drugs are possible. Tell your doctor, nurse, or
pharmacist about all the medicines you take,
including drugs prescribed by other doctors,
vitamins, minerals, natural supplements, or
alternative medicines.
The following may interact with TEVA-COMBO
Sterinebs®
:
- digitalis;
- other anticholinergic drugs, such as ipratropium bromide and other beta2- adrenergic agents such as salbutamol, the individual ingredients of TEVA-COMBO Sterinebs® ;
- beta blockers, such as propranolol;
- xanthine derivatives such as theophylline;
- monoamine oxidase inhibitors such as isocarboxazid
- tricyclic antidepressants such as amitriptyline;
- epinephrine;
- certain diuretics or “water pills” such as furosemide, hydrochlorothiazide.
Proper use of this medication
TEVA-COMBO Sterinebs®
should only be inhaled from a
nebulizer. It must not be injected or swallowed.
Do not let the TEVA-COMBO Sterinebs®
or the mist
produced by the nebulizer, get in your eyes.
Use your nebulizer in a well ventilated room. Some
of the mist will be released into the air and may be
breathed in by others.
Use TEVA-COMBO Sterinebs®
only as directed by your
doctor. During administration your doctor may want
to monitor your blood.
Treatment with TEVA-COMBO Sterinebs®
is to be
initiated and administered under medical supervision
(e.g. in the hospital setting). Home based treatment
can be recommended in exceptional cases (severe
symptoms or experienced patients requiring higher
doses) when a low dose rapid acting beta-agonist
bronchodilator has been insufficient in providing
relief after consultation with an experienced
physician. Administration should be stopped when
sufficient symptom relief is achieved.
TEVA-COMBO Sterinebs®
has been prescribed to treat
your current condition. DO NOT give it to other
people. Always use TEVA-COMBO Sterinebs®
exactly as
your doctor has told you. You should check with
your doctor or pharmacist if you are not sure.
TEVA-COMBO Sterinebs®
should be used only in a
properly functioning and regularly maintained
nebulizer or an intermittent positive pressure
ventilator. Before starting treatment, be certain
that you are completely familiar with the use and
proper care of your nebulizer. The content of the
unit dose vials does not need to be diluted for
nebulization.
Not recommended for use in children and adolescents
under 18 years of age.
Usual Adult Dose
The recommended dosage is 1 unit dose vial (0.50
mg
ipratropium bromide (as ipratropium bromide
monohydrate) and 2.5 mg salbutamol (as salbutamol
sulfate) in 2.5 mL) three or four times daily.
If one unit dose vial does not improve your
breathing difficulties, you may need another unit
dose vial. If this is the case, you should contact
your doctor or the nearest hospital.
Your doctor or pharmacist will tell you how to
prepare your
TEVA-COMBO Sterinebs®
solution for inhalation. Your
doctor or pharmacist might instruct you to use
sterile sodium chloride solution (0.9%) to dilute
the TEVA-COMBO Sterinebs®
. If you are told to
dilute TEVA-COMBO Sterinebs®
solution, you must do
so immediately before you plan to use the
solution. If necessary, doses may be diluted to a
total nebulization volume of 3-5 mL with
preservative free 0.9% sterile sodium chloride
solution and used immediately. Discard any unused
solution. Nebulize over 10-15 minutes at gas flow
of 6-10L/min. Repeat every six hours as necessary.
Please read and carefully follow these
instructions:
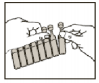
Open the pouch foil and detach one plastic vial by pulling it firmly from the strip.-
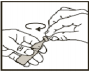
Open the vial by twisting off the top. It is important that you use the contents of the vial as soon as possible after opening it. -
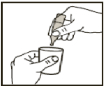
Squeeze the contents of the plastic vial into your nebulizer chamber. If your doctor has instructed you to use less than one complete vial, use a syringe to withdraw the prescribed dose. Any solution left in the plastic vial must be thrown away. 
Using a syringe, add sodium chloride solution to the chamber if you have been directed to do so by your pharmacist or physician.-
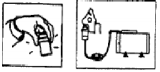
Gently shake the nebulizer chamber and connect it to the mouthpiece or face mask. Then connect the nebulizer tube to the air or oxygen pump and begin therapy. -

Breathe calmly and deeply through the mask or mouthpiece until no more mist is formed in the nebulizer chamber. This usually takes 10-15 minutes.
It is very important to adjust the face mask, if required, to prevent the mist from getting in your eyes. - Follow the instructions provided by the nebulizer and air pump manufacturers for the proper care and maintenance of the equipment. Keep the nebulizer, nebulizer tube and face mask clean to minimize microbial contamination.
Do not mix TEVA-COMBO Sterinebs® with other drugs in the same nebulizer.
Overdose
Missed Dose
If a dose is missed and no symptoms occur, the regular next dose according to the dosing schedule should be taken. If a dose is missed and respiratory symptoms are experienced, the missing dose should be taken and the dosing schedule according to the recommended dosage should be resumed.
Side effects and what to do about them
Side effects may include:
- wheezing after inhalation;
- headache, dizziness;
- nausea (feeling sick), digestive problems like constipation, diarrhoea and vomiting;
- muscle problems such as cramps weakness, pain, feeling weak, tremor (shaking);
- feeling nervous;
- mental disorder;
- impaired voice sounds;
- increased sweating;
- bronchitis and upper respiratory tract infection (a cold)
- throat irritation, cough, dry mouth or throat, bad taste-sucking on a sour candy or rinsing your mouth may help.
TEVA-COMBO Sterinebs® contains a beta-agonist, and taking additional doses in the form of other single agent, beta-agonists (fenoterol, salbutamol etc.) could cause harmful effects on the heart, lungs and circulatory system. Therefore do not take additional bronchodilators by inhalation with TEVA-COMBO Sterinebs® unless instructed to do so by your doctor or pharmacist.
Stop taking the medication and tell your doctor immediately if you notice any of the following:
- you are wheezy or have any other difficulties in breathing;
- you are having an allergic reaction – the signs may include skin rash, itching and nettle rash. In severe cases the signs include swelling of your tongue, lips and face, sudden difficulties in breathing and reduction of your blood pressure.
If you have any questions about TEVA-COMBO Sterinebs® or your nebulizer, contact your doctor or pharmacist.
| Symptom / effect | Talk with your doctor, nurse, or pharmacist only if severe | Talk with your doctor, nurse, or pharmacist in all cases | Stop taking drug and seek immediate medical help |
|---|---|---|---|
| Uncommon | |||
| Bronchospasm: Increased wheezing or tightness in the chest, difficulty in breathing, coughing bouts | ✔ | ||
| Shortness of breath | ✔ | ||
| Hypotension or Hypertension, Changes in blood pressure: dizziness, fainting, lightheadedness | ✔ | ||
| Skin rash | ✔ | ||
| Rare | |||
| Allergic Reaction: rash, hives, swelling of the face, lips, mouth, tongue or throat, difficulty swallowing or breathing, choking due to swelling of the muscles around the voice box | ✔ | ||
| Fast or irregular heart beat / chest pain | ✔ | ||
| Eye Disorders: new or worsened pressure in your eyes, eye pain or discomfort, blurred vision, seeing halos or rainbows around items or red eyes | ✔ | ||
| Urinary Retention: difficulty and pain when passing urine, urinating frequently, urination in a weak stream or drips | ✔ | ||
| Muscle pain, weakness or spasms; paralysis | ✔ | ||
| Myocardial Ischaemia: decreased blood flow to the heart leading to angina (chest pain), shortness of breath, or a heart attack | ✔ | ||
| Angina: Chest pain | ✔ | ||
| Decreased levels of potassium in the blood: irregular heartbeats, muscle weakness and generally feeling unwell | ✔ | ||
This is not a complete list of side effects. For any unexpected effects while taking TEVA-COMBO Sterinebs® , contact your doctor or pharmacist.
How to store
Unopened unit dose vials of TEVA-COMBO Sterinebs®
should be stored at room temperature (15-25°C).
The vials should be protected from heat and light.
Do not use if solution is discoloured. Keep out
of reach of children.
Partly used, opened or damaged unit dose vials
should be discarded.
Reporting side effects
You can report any suspected adverse reactions associated with the use of health products to the Canada Vigilance Program by one of the following three ways:
- Report online at www.healthcanada.gc.ca/medeffect
- Call toll-free at 1-866-234-2345
- Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-866-678-6789, or
- Mail to:
Canada Vigilance Program
Health Canada
Postal Locator 0701E
Ottawa ON K1A 0K9
Postage paid labels, Canada Vigilance Reporting Form and the adverse reaction reporting guidelines are available on the MedEffectTM Canada Web site at www.healthcanada.gc.ca/medeffect.
NOTE: Should you require information related to the management of side effects, contact your health professional. The Canada Vigilance Program does not provide medical advice.
More information
This document plus the full product monograph, prepared for
health professionals can be found by contacting
Teva Canada Limited
at: 1-800-268-4127 ext. 1255005 (English)
or 1-877-777-9117 (French)
or druginfo@tevacanada.com
This leaflet was prepared by:
Teva Canada Limited
30 Novopharm Court
Toronto, Ontario
Canada, M1B 2K9
Last revised: March 1, 2016