Details for: MONOPROST
Company: LABORATOIRES THEA
| DIN | DIN name | Active Ingredient(s) | Strength | Dosage Form | Route of Administration |
|---|---|---|---|---|---|
| 02456230 | MONOPROST | LATANOPROST | 50 MCG / ML | SOLUTION | OPHTHALMIC |
Summary Reports
Consumer Information
This information was provided by the drug’s manufacturer when this drug product was approved for sale in Canada. It is designed for consumers and care givers. It is a summary of information about the drug and will not tell you everything about the drug. Contact your doctor or pharmacist if you have any questions about the drug.
What the medication is used for
MONOPROST is used to treat ocular hypertension (high pressure in the eye) in patients with open-angle glaucoma or ocular hypertension. These conditions may eventually affect your eyesight.
What it does
MONOPROST is a preservative-free solution for use only in the eyes. The active ingredient in MONOPROST belongs to a group of medicines known as prostaglandins. It lowers the pressure within your eye by increasing the natural outflow of fluid from inside the eye.
When it should not be used
Do not use MONOPROST:
- If you are allergic (hypersensitive) to latanoprost or any of the other ingredients of this medicine (see non-medicinal ingredients).
What the medicinal ingredient is
Latanoprost.
What the non-medicinal ingredients are
Macrogolglycerol hydroxystearate 40, sorbitol, carbomer 974P, macrogol 4000, disodium edetate, sodium hydroxide (for pH-adjustment), water for injection.
What dosage form it comes in
MONOPROST is available in single-dose containers packed in a sachet of 5 units. A pack size contains 30 (6 x 5) or 90 (18 x 5) single-dose containers.
Warnings and precautions
Talk to your doctor or pharmacist before using MONOPROST if you think any of the following apply to you:
- If you are about to have or have had eye surgery (including cataract surgery).
- If you suffer from eye problems (such eye pain, irritation or inflammation, blurred vision).
- If you have liver or kidney problems
- If you know that you suffer from dry eyes.
- If you have severe asthma or your asthma is not well controlled, or you have chronic obstructive pulmonary disease (COPD).
- If you suffer from eye problems such as eye pain, irritation, inflammation (e.g. uveitis, iritis) or blurred vision.
- If you wear contact lenses. You can still use MONOPROST, but follow the instruction for contact lens in PROPER USE OF THIS MEDICATION.
- If you have suffered or are currently suffering from a viral infection of the eye caused by the herpes simplex virus (HSV).
Children: MONOPROST has not been investigated in children (below 18 years) and is not recommended for use in children.
Pregnancy and breast-feeding: Ask your doctor for advice before using MONOPROST if you:
- are pregnant or plan to become pregnant
- are breast-feeding or plan to breast-feed
Driving and using machines: When you use MONOPROST you might have blurred vision, for a short time. If this happens to you, do not drive or use any tools or machines until your vision becomes clear again.
Important information about some of the ingredients: MONOPROST contains macrogolglycerol hydroxystearate (derived from castor oil) which may cause skin reactions.
Interactions with this medication
MONOPROST may interact with other medicines. Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Mixing other eye drops containing thiomersal with MONOPROST may result in precipitation (solids appearing) in the drops. If these drugs or other eye drops are used, allow at least 5 minutes between the application of each medicine.
Let your doctor know if you are taking other prostaglandin products.
Proper use of this medication
Usual dose
- Always use MONOPROST exactly as your doctor has advised you, until your doctor tells you to stop. Check with your doctor or pharmacist if you are not sure.
- The usual adult dose (including the elderly) is one drop once a day in the affected eye(s). The best time to do this is in the evening.
- Do not use MONOPROST more than once a day, because the effectiveness of the treatment can be reduced if you administer it more often.
Contact lens wearers: If you wear contact lenses, you should remove them before using MONOPROST. After using MONOPROST you should wait 15 minutes before putting your contact lenses back in.
Instructions for use: The drops are supplied in single-dose
containers. The solution from one individual single dose
container of MONOPROST is to be used immediately after
opening for administration to the affected eye(s). Since
sterility cannot be maintained after the individual single dose
container is opened, a new container must be opened prior to
each use and must be discarded immediately after
administration.
Please wash your hands then sit or stand comfortably before
following these instructions to use the drops:
- Open the container using your fingers from one hand to hold the body of the container. Using the fingers from the other hand hold the top of the container. With a snapping motion break the top off the container.
- Use your finger to gently pull down the lower eyelid of
your affected eye.
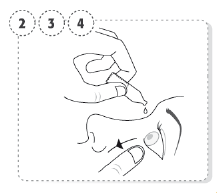
- Place the tip of the single-dose container close to, but not touching your eye. Avoid contact between the container tip and the eye and eyelid.
- Squeeze the single-dose container gently so that only one drop goes into your eye and then release the lower eyelid.
- Press a finger against the corner of the affected eye by
the nose. Hold for 1 minute whilst keeping the eye
closed.
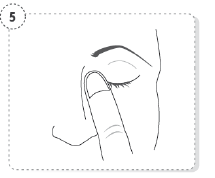
- Repeat in your other eye if your doctor has told you to do this.
- Discard the single-dose container after use. Do not keep it to use it again.
Contact your doctor or pharmacist as soon as possible if you
swallow MONOPROST accidentally.
Using MONOPROST with other eye drops: Wait at least 5
minutes between using MONOPROST and using other eye
drops.
If you stop using MONOPROST: You should speak to your
doctor if you want to stop using MONOPROST.
If you have any further questions on the use of this medicine,
ask your doctor or pharmacist.
Overdose
If you put too many drops into your eye, you may experience some minor irritation in your eye and your eyes may water and turn red, this should pass, but if you are worried contact your health care practitioner for advice.
Missed Dose
If you forget to use MONOPROST wait until it is time to take the next dose at the usual time. Do not take more than one dose. If you are unsure about anything talk to your doctor or pharmacist.
Side effects and what to do about them
Like all medicines, this medicine can cause side effects,
although not everybody gets them.
MONOPROST may cause:
- A gradual change in your eye colour by increasing the
amount of brown pigment in the coloured part of the eye
known as the iris.
- If you have mixed-colour eyes (blue-brown, greybrown, yellow-brown or green-brown) you are more likely to see this change than if you have eyes of one colour (blue, grey, green or brown eyes).
- Changes in eye colour may not noticeable for several months to years.
- The colour change may be permanent and may be more noticeable if you use MONOPROST in only one eye.
- This colour change is not known to be associated with any symptom of pathological change.
- The eye colour change does not continue after MONOPROST treatment is stopped.
- Redness of the eye.
- Eye irritation (a feeling of burning, grittiness, itching, stinging or the sensation of a foreign body in the eye).
- A gradual change to eyelashes of the treated eye and the fine hairs around the treated eye. These changes involve an increase of the colour (darkening), length, thickness and number of your eye lashes.
Common side effects in the eye:
- Burning, stinging, red eyes, eye pain, dry eyes, instillation pain/itching, excess tearing
- Feeling something is in the eye, blurred vision, sensitivity to light,
- Staining or damage to the surface of the cornea
Uncommon side effects in the eye:
- Double vision, eye discharge, conjunctivitis, eyelid inflammation
- Iritis or uveitis (inflammation of the interior of the eye)
- Inflammation of the cornea
Common side effects in the body:
- muscle and joint pain, bronchitis, sinusitis, skin rash
If you get any side effects, talk to your doctor or, pharmacist or nurse. This includes any side effects not listed in this leaflet.
This is not a complete list of side effects. For any unexpected effects while taking MONOPROST, contact your doctor or pharmacist.
How to store
Store MONOPROST between 15 and 25°C and protect from light.
Do not use this medicine after the expiry date which is stated on the carton, sachet and single-dose container.
After opening the sachet of 5 single-dose containers, use the containers within 7 days.
After first opening the single-dose container, use immediately and discard the single-dose container after use.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
Keep all medicines in a safe place, out of the reach and sight of children.
Reporting side effects
You can report any suspected adverse reactions associated with the use of health products to the Canada Vigilance Program by one of the following 3 ways:
- Report online at www.healthcanada.gc.ca/medeffect
- Call toll-free at 1-866-234-2345
- Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-866-678-6789, or
- Mail to: Canada Vigilance Program
Health Canada
Postal Locator 0701D
Ottawa, Ontario
K1A 0K9
Postage paid labels, Canada Vigilance Reporting Form and the adverse reaction reporting guidelines are available on the MedEffectTM Canada Web site at www.healthcanada.gc.ca/medeffect.
NOTE: Should you require information related to the management of side effects, contact your health professional. The Canada Vigilance Program does not provide medical advice.
More information
This document plus the full product monograph, prepared for health professionals can be obtained by contacting the sponsor, Laboratoires THEA, 12 rue Louis Blériot 63017 Clermont-Ferrand Cedex 2 – FRANCE.
1-800-XXX-XXXX
Imported and distributed by
Labtician Ophthalmics, Inc.
2140 Winston Park Drive, Unit 6
Oakville, ON L6H 5V5
This leaflet was prepared by Laboratoires Thea.
Last revised: July 6, 2016