Details for: TESTIM 1%
Company: PALADIN LABS INC.
| DIN | DIN name | Active Ingredient(s) | Strength | Dosage Form | Route of Administration |
|---|---|---|---|---|---|
| 02280248 | TESTIM 1% | TESTOSTERONE | 1 % | GEL | TRANSDERMAL |
Summary Reports
Consumer Information
This information was provided by the drug’s manufacturer when this drug product was approved for sale in Canada. It is designed for consumers and care givers. It is a summary of information about the drug and will not tell you everything about the drug. Contact your doctor or pharmacist if you have any questions about the drug.
What the medication is used for
Your doctor has prescribed Testim® 1% because your body is not making enough testosterone. The medical term for this condition is hypogonadism.
What it does
Testim® 1% delivers medicine into your bloodstream through your skin. Testim® 1% helps raise your testosterone to normal levels.
When it should not be used
- If you have or it is suspected that you have prostate or breastcancer.
- Known allergy to any of its components [the activeingredient is testosterone, which may be synthesized fromsoy; (see "What the nonmedicinal ingredients are" in thissection)]
Testim® 1% should NOT be used by women. Pregnant and breast feeding women are especially at risk and should avoid skin contact with application sites on men. Testosterone may cause harm to your unborn baby. Testosterone exposure during pregnancy has been reported to be associated with fetal abnormalities. If skin contact with unwashed or unclothed application sites of men using Testim® 1% or with clothing or other fabrics exposed to Testim® 1% occurs, pregnant or nursing women should immediately wash the area of contact with soap and water.
What the medicinal ingredient is
Testosterone
What the non-medicinal ingredients are
Carbomers, ethanol, glycerol, pentadecalactone, polyethylene glycol, propylene glycol, purified water, trometamol
What dosage form it comes in
Each Testim® 1 % 5 g tube contains testosterone 1% in a clear to translucent hydroalcoholic topical gel. Testim® 1% is supplied in unit-dose aluminum tubes with epoxy phenolic liners in cartons of 30.
Warnings and precautions
Serious Warnings and Precautions
1% can be transferred to another person when skin-to-skin contact with application site occurs.
- Signs of puberty (unexpected sexual development) havebeen reported in children who were exposed totestosterone gel.
- Keep children away from unwashed or unclothedapplication sites of men using Testim® 1% and fromunwashed clothing or other fabrics exposed to Testim®1%.
- Men who use Testim® 1% must follow the instructionsfor use to lower the risk of transferring Testim® 1% toanother person.
You should prevent Testim® 1% from transferring to another person, especially pregnant or breast feeding women, or children by taking the following precautions:
- Children and women should avoid contact with theapplication sites on men using Testim®1%.
- Testim® 1% should be applied only to the areas of theshoulders and/or upper arms that will be covered by a shortsleeve T-shirt.
- Direct skin-to-skin contact should be avoided after applyingTestim®1%. If direct skin-to-skin contact is anticipated, washthe application site(s) thoroughly with soap and water toremove any Testim®1% left on the application site(s).
- Wash hands immediately with soap and water after applicationof Testim®1%;
- Cover the application site(s) with clothing (such as a shirt)after Testim®1% has dried;
- In the event that an unwashed or uncovered Testim® 1%application site does come in direct contact with the skin ofanother person, the general area of contact on the other personshould be washed with soap and water as soon as possible.
In children, signs of testosterone exposure can include unexpected sexual development such as inappropriate enlargement of the penis or clitoris, premature development of pubic hair, increased erections, or aggressive behaviour. In women, signs of testosterone exposure include changes in body hair distribution, significant increase in acne, or other signs of the development of masculine traits. Any of these changes should be brought immediately to the attention of a doctor. The possibility of exposure to testosterone should be discussed with the doctor.
Testim® 1% must not be used by children under the age of 18.
There is very little information from clinical trials with testosterone in the older male (over 65 years of age) to support safe use for a long period of time.
You should not use testosterone in an attempt to reduce weight and increase muscle, or improve athletic performance as it may cause serious health problems.
You should not use testosterone to treat sexual dysfunction or male infertility.
Before using Testim® 1%, talk to your doctor if you:
- have difficulty urinating due to an enlarged prostate. Olderpatients may have a higher risk of developing an enlarged prostate or prostate cancer;
- have prostate cancer (confirmed or suspected);
- have liver, kidney or heart disease;
- have high blood pressure (hypertension);
- have diabetes;
- have breathing problems during sleep (sleep apnea);
- have heart or blood vessel problems or a history of theseproblems such as heart attacks, stroke, or blood clot in thelungs or legs.
Drug Abuse and Dependence:
Testim® 1% contains testosterone, which is a controlled substance under Schedule G of the Food and Drugs Act.
Precautions while using Testim® 1%:
Following application of Testim® 1%, allow gel to dry completely before smoking or going near an open fire.
Interactions with this medication
Be sure to inform your doctor or pharmacist if you are taking or have recently taken any other drugs or herbal products, even those without a prescription.
Drugs that may interact with Testim® 1% include:
- Insulin
- Corticosteroids
- Propranolol
- Anti-clotting medications (such as warfarin)
Proper use of this medication
Always use Testim® 1% as your doctor has instructed you.
Usual dose
The recommended starting dose of Testim® 1% is 5 g of gel (one tube) containing 50 mg of testosterone. The gel should be applied once daily (preferably in the morning) to clean, dry intact skin and only to the areas of the shoulders and/or upper arms that will be covered by a short sleeve T-shirt. Some patients will require a higher dose and your doctor may prescribe two tubes of gel every day.
Instructions for Use:
- Apply Testim® 1% at the same time each day (preferablyevery morning).
- If you take a bath or shower in the morning, use Testim® 1%after your bath or shower.
- Make sure that your skin is clean and completely dry beforeputting on the gel.
- Open the Testim® 1% aluminum tube by piercing the end ofthe tube. Use the top part of the cap to do this.
- Squeeze the entire contents of the tube into the palm of yourhand. Squeeze the gel out from the bottom of the tube towards the top. If you prefer, you can squeeze out a portion at a time and continue until the tube is empty.
- Apply Testim® 1% only to the areas of your shoulders and/orupper arms that will be covered by a short sleeve T-shirt. In this way your body will absorb the right amount of testosterone. Never apply Testim® 1% to your genitals (penis and scrotum), sunburned areas of the body, or to skin that is not completely normal. Apply Testim® 1% only to healthy, normal skin. Avoid contact with open sores, wounds or irritations.
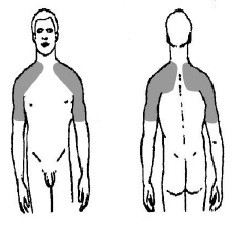
- Use a circular motion to rub the gel in to your skin for severalseconds. Do this until the gel is well rubbed in and the skinarea feels dry.
- Thoroughly wash your hands with soap and waterimmediately after application to reduce the chance that themedicine will spread from your hands to other people.
- Let Testim® 1% dry for a few minutes before you dress. Thisprevents your clothing from wiping the gel off your skin andensures that your body will absorb the correct amount oftestosterone.
- Washing the application site up to 2 hours after application ofTestim® 1% can lower the amount of testosterone that isabsorbed by your body, although levels should stay within thenormal range. Therefore, you should wait at least two (2)hours after applying the gel before showering or swimming toensure that the greatest amount of Testim® 1% is absorbedinto your body. On rare occasions, you may shower or swimas soon as one hour after applying Testim® 1%. If doneinfrequently, this will have little effect on the amount ofTestim® 1% that is absorbed by your body.
- If you expect that the area of skin to which you applied Testim® 1% may come into direct skin contact with someone else, you should either thoroughly wash the application site with soap and water before that encounter or keep it covered with clothing. This will reduce the chance that the medicine will transfer to the other person.
- The used tubes of Testim® 1% should be discarded in household waste in a manner that prevents accidental exposure of children or pets.
What should I do if I get Testim® 1% in my eyes?
If you get Testim® 1% in your eyes, rinse your eyes right away with warm clean water to flush out any Testim® 1%. Seek medical attention if discomfort persists.
Missed Dose
If you miss a dose, do not double your next dose the next day to catch up. If your next dose is less than 12 hours away, it is best to wait. Do not take the skipped dose. If it is more than 12 hours until your next dose, take the dose that you missed. Resume your normal dosing the next day.
Overdose
Contact your doctor or pharmacist immediately if you suspect an overdose.
If you use more Testim® 1% than the recommended dose (an overdose), wash the skin with soap and water where Testim® 1% was applied and contact your doctor or pharmacist.
What to do if someone else is exposed to the medication:
If someone else is exposed to Testim® 1% either by direct contact with the gel itself or indirectly because of contact with your treated
skin, that person should wash his or her area of contact thoroughly with soap and water as soon as possible. The longer the gel is in contact with the skin before washing, the greater is the chance that the other person will absorb some testosterone.
This is particularly important for women, especially pregnant or nursing women, and children. Children have naturally low levels of testosterone and could be harmed by higher levels.
Pregnant women are at an even higher risk because increased testosterone levels may cause harm or abnormalities in the unborn baby.
Never share Testim® 1% with anyone.
Side effects and what to do about them
Like all medicines, Testim® 1% can have side effects. The following side effects have been reported for products containing testosterone:
- Skin irritation or redness or rash at the application site;
- increased prostatic specific antigen (PSA);
- enlarged prostate (benign prostatic hyperplasia);
- an increase in red blood cell count, (hematocrit andhemoglobin);
- acne;
- change in mood, depression;
- prolonged or painful erection;
- sleep disturbances caused by breathing problems;
- aggression or aggressive behaviour;
- breast enlargement and breast pain;
- loss of hair and baldness;
- high blood pressure;
- weight gain;
- headache, dizziness;
- increased or irregular heart rate, blood clot in the lungs or thelegs.
Signs of puberty (unexpected sexual development) have been reported in children who were exposed to testosterone gel. See WARNINGS ANDPRECAUTIONS.
Changes in body hair distribution, significant increase in acne, or other signs of the development of masculine traits in the female partner or in any person (including children) exposed to skin-to- skin contact, should be brought to the attention of a doctor.
This is not a complete list of side effects. For any unexpected effects while taking Testim® 1%, contact your doctor or pharmacist.
| Symptom / effect | Talk with your doctor, nurse, or pharmacist only if severe | Talk with your doctor, nurse, or pharmacist in all cases | Stop taking drug and talk with your doctor or pharmacist |
|---|---|---|---|
| Common | |||
| Urinary symptoms (i.e. change in frequency/color, dribbling, pain on urination, straining, weak stream, small amounts) | ✔ | ||
| Uncommon (after prolonged use) | |||
| Breast enlargement or breast pain | ✔ | ||
| Uncommon | |||
| Swelling of ankles and legs (in patients with heart, kidney or liver damage) | ✔ | ||
| Erections that are too frequent or continue for too long | ✔ | ||
| Heart attack and stroke | ✔ | ||
| Uncommon (Seen with long term, high dose testosterone treatments) | |||
| Liver problems, with symptoms such as nausea, vomiting, along with yellowed or darkened skin | ✔ | ||
How to store
Store at Room Temperature (15°C – 30°C).
Keep in a safe place out of reach of children and pets.
Reporting side effects
To monitor drug safety, Health Canada through the CanadaVigilance Program collects information on serious andunexpected side effects of drugs. If you suspect you have had aserious or unexpected reaction to this drug you may notifyCanada Vigilance.
You can report any suspected adverse reactions associated withthe use of health products to the Canada Vigilance Program byone of the following 3 ways:
- Report online at www.healthcanada.gc.ca/medeffect
- Call toll-free at 1-866-234-2345
- Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-866-678-6789, or
- Mail to: Canada Vigilance Program
Health Canada
Postal Locator 0701C
Ottawa, On K1A 0K9
Postage paid labels, Canada Vigilance Reporting Formand theadverse reaction reporting guidelines are available on the MedEffectTM CanadaWeb site at www.healthcanada.gc.ca/medeffect.
NOTE: Should you require information related to themanagement of side effects, contact your health professional.The Canada Vigilance Program does not provide medicaladvice.
More information
This document plus the full product monograph, prepared for health professionals can be found on the sponsor website www.paladinlabs.com or by calling at: 1-888-867-7426
This leaflet was prepared by Paladin Labs Inc.
Last revised: February 3, 2017.