Details for: GRASTEK
Company: ALK-ABELLO A/S
| DIN | DIN name | Active Ingredient(s) | Strength | Dosage Form | Route of Administration |
|---|---|---|---|---|---|
| 02418304 | GRASTEK | STANDARDIZED TIMOTHY GRASS POLLEN | 2800 UNIT | TABLET (ORALLY DISINTEGRATING) | SUBLINGUAL |
Consumer Information
This information was provided by the drug’s manufacturer when this drug product was approved for sale in Canada. It is designed for consumers and care givers. It is a summary of information about the drug and will not tell you everything about the drug. Contact your doctor or pharmacist if you have any questions about the drug.
What the medication is used for
GRASTEK® is used for the treatment of adults and children 5 years of age and older with a history of allergy to specific grass pollen. Grass pollen allergy is characterized by rhinitis (sneezing, runny or itchy nose, nasal congestion) with or without conjunctivitis (itch and/or watery eyes).
Before you begin treatment with GRASTEK®, your allergy will be confirmed by a physician who will perform skin and/or blood tests.
GRASTEK® has not been tested in patients younger than 5 year or older than 65 years of age.
What it does
GRASTEK® is an allergy tablet that reduces symptoms due to specific grass allergens. It contains an allergen extract that helps to make you less sensitive to the grass pollens you are allergic to.
When it should not be used
Do not take GRASTEK® if you or your child:
- Have severe or difficult-to-control asthma;
- Are allergic (hypersensitive) to any of the other ingredients of GRASTEK® (see What the non- medicinal ingredients are);
- Have had a serious allergic reaction to grass pollen allergy shots, tablets or drops;
- Are taking beta-blockers (a medicine prescribed for heart conditions, such as high blood pressure);
- Have any swelling or sores in your mouth
What the medicinal ingredient is
The active substance is Standardized Allergen Extract of Timothy Grass (Phleum pratense)
What the non-medicinal ingredients are
fish gelatin, mannitol, sodium hydroxide. GRASTEK® does not contain lactose.
What dosage form it comes in
GRASTEK® is a prescription tablet that you place under your tongue once daily.
Each tablet has a strength of 2800 Bioequivalent Allergy Units (BAU).
Warnings and precautions
Serious Warnings and Precautions
- GRASTEK® is intended for use only by physicians with adequate training and experienced in the treatment of allergic diseases. With prescription for children, the doctor should also have relevant experience in treating children.
- It is common for patients to experience mild or moderate local allergic reactions with GRASTEK® (for example, an itchy mouth or a sore throat). Serious allergic reactions which can be life-threatening have happened in patients treated with GRASTEK®. If you experience stronger allergic reactions with a feeling of swelling in the throat, difficulty swallowing or breathing and voice changes, contact your physician immediately. The treatment has to be stopped immediately until your physician advises otherwise.
- The first tablet of GRASTEK® must be taken at the doctor’s office. Your doctor will also tell you to stay on site for 30 minutes to check out for possible side effects to the treatment you may have.
- Children must be watched by an adult for at least 30 minutes after each dose.
Stop treatment with GRASTEK® and contact your doctor if you have any of the following symptoms that do not go away or that worsen: heartburn, difficulty swallowing, pain with swallowing, or chest pain
Before you take GRASTEK®, tell your doctor if you or your child:
- have ever had a serious allergic reaction to allergy shots, tablets or drops.
- have worsening asthma symptoms or breathing problems.
- have recently had any mouth injury or mouth surgery (such as a tooth removal).
- are pregnant or plan to become pregnant.
- are breast-feeding or plan to breast-feed. It is not known if GRASTEK® will pass into breast milk.
- have diseases affecting the immune system e.g. autoimmune diseases, immune complex diseases or (severe) immune deficiency diseases.
- have malignant diseases (e.g. cancer).
Interactions with this medication
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Your doctor will tell you if it is safe to take other medicines while you are using GRASTEK®. No drug interaction studies have been done in patients.
Proper use of this medication
The first dose of GRASTEK® should only be taken in the doctor’s office. After taking the first dose, you or your child must be watched for 30 minutes by a healthcare professional for symptoms of a serious allergic reaction.
- Your child should only be given each dose of GRASTEK® by an adult.
- Your doctor may prescribe medicines for you or your child to take should you have a serious allergic reaction.
After the first dose, you or your child may take GRASTEK® at home. GRASTEK® should only be given to your child under adult supervision.
Usual Dose
GRASTEK® treatment can begin at any time during the year. For symptom improvement during the first grass pollen season, you should start taking GRASTEK® at least 8 to 12 weeks before the grass pollen season usually begins. Take GRASTEK® once daily for as long as your doctor tells you to take it, usually until at least the end of the yearly grass pollen season.
How should I take GRASTEK®?
- Do not use food or water to take the tablet.
- Remove the tablet from the package with dry hands by carefully removing the foil.
- Place the tablet under the tongue right away. It will dissolve.
- Do not swallow for about 1 minute.
- Do not drink or eat for 5 minutes after taking the tablet.
- Wash your hands after handling the tablet.
Detailed Instructions
- Tear off the strip marked with triangles at the top of the blister pack.
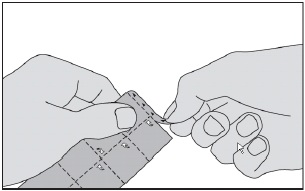
- Tear a square off the blister pack along the perforated lines.
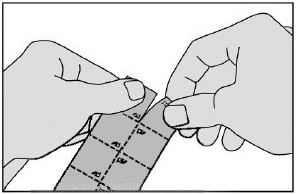
- Remove the tablet carefully from the foil (do not force the tablet through the foil. It may become damaged as it easily breaks. Instead, fold back the marked corner of the foil and then pull it off). Take it immediately.
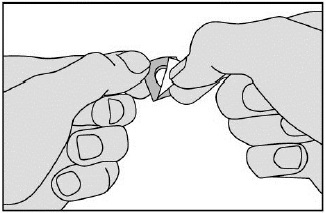
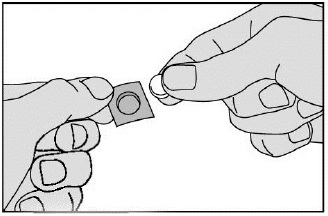
- Place the tablet under the tongue. Allow it to remain there for a few seconds until it dissolves. Do not swallow during the first minute. Do not eat or drink for 5 minutes. Wash hands after handling the tablet.

General information about the safe and effective use of
GRASTEK®
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
If necessary, your physician may at the same time prescribe medications to treat the possible allergic reactions due to GRASTEK® treatment.
Missed Dose or Interruption of Treatment
If you miss a dose, return to your normal schedule the next day. Do not take a double dose to make up for forgotten dose.
Overdose
- Do not take more than one GRASTEK® tablet daily.
- Do not take a double dose to make up for forgotten dose.
Side effects and what to do about them
Like all medicines, GRASTEK® can cause side effects, although not everybody gets them. The side effects usually happen during the first days or weeks of treatment and go away on their own.
The most common side effects of GRASTEK® include:
- itching or swelling of the mouth
- throat irritation
- itching of eyes, nose and throat
Uncommonly, stronger allergic reactions to GRASTEK® can occur, such as swelling of the throat, difficulty swallowing or breathing, and voice changes. If you experience these symptoms, contact your physician or pharmacist immediately, and do not take any more doses until being assessed by your doctor.
The following side effects were reported by adults or by children and adolescents who were treated with GRASTEK® in clinical studies:
Very common (seen in at least 1 in 10 patients):
outh itching and/or swelling and/or inflammation, irritation in the throat, swelling in the mouth.
Common (seen in at least 1 in 100 patients, but in less than 1 in 10 patients):
Mouth: Mouth tingling and/or numbness and/or pain, and/or discomfort and/or redness, inflammation and/or burning, decreased sensation, dryness, blisters, itching of the tongue and/or lips, swelling in the lips and/or tongue and/or throat or the roof of the mouth, inflammation and/or burning sensation in the tongue, pain in the gums. Throat: tightness, difficulty swallowing, enlarged glands, pain and/or swelling and/or redness, blisters, dryness. Nose: sneezing, runny nose, dryness, irritation inside the nose, congestion, uncomfortable feeling in the nose. Eyes: itching and/or irritation, watery eyes. Other: headache, chest pain and/or discomfort, rapid heartbeat, shortness of breath, cough, dizziness, abnormal skin sensation, nausea, upset stomach, fatigue, itching, itch rash, hives, change in voice, sensation of a foreign body, sudden redness of the skin, excessive sensitivity.
Uncommon (seen in at least 1 in 1,000 patients, but in less than 1 in 100 patients:
A feeling that the ear is blocked, uncomfortable feeling in the ear, ear pain, eye pain, inflammation of the eye, swelling of the eye, irritation of the eyelid, redness of the eye, oral pain, salivary gland enlargement, stomach pain, diarrhea, acid stomach, bleeding of the gums, inflammation of the tongue, blistering of the lip and mouth, pain with swallowing, oral discomfort, sensitive teeth, excessive salivating, vomiting, pain, stomach virus, mouth abscess, skin infection around the eye, upper respiratory infection, loss of appetite, sleepiness, burning sensation, sinus headache, asthma, asthma caused by exercise, nose bleed, obstruction in the nose, wheezing, swelling of the tonsils, skin rashes, redness of the skin, excessive sweating.
The following side effect was reported with general use: Eosinophilic esophagitis (EoE) which may present as any of the following symptoms that do not go away or worsen: heartburn, difficulty swallowing, pain with swallowing, or chest pain.
The following additional side effects have been reported with GRASTEK®:
- high-pitched unusual breathing sound that may be a sign of trouble breathing
| Symptom / effect | Talk with your doctor, nurse, or pharmacist only if severe | Talk with your doctor, nurse, or pharmacist in all cases | Stop taking drug and talk with your doctor or pharmacist |
|---|---|---|---|
| Very common | |||
| Swelling in the mouth | ✔ | ||
| Common | |||
| Swollen tongue | ✔ | ||
| Swelling in the throat | ✔ | ||
| Trouble swallowing | ✔ | ||
| Chest discomfort | ✔ | ||
| Hives all over your body | ✔ | ||
| Itching all over your body | ✔ | ||
| Throat tightness | ✔ | ||
| Trouble breathing | ✔ | ||
| Rare | |||
| Severe allergic reactions or asthma Shortness of breath |
Seek emergency help immediately | ||
How to store
- Store at room temperature (15° to 30°C).
- Store GRASTEK® in the original package and protect from moisture.
- Keep out of reach and sight of children.
Reporting side effects
You can help improve the safe use of health products for Canadians by reporting serious and unexpected side effects to Health Canada. Your report may help to identify new side effects and change the product
safety information.
3 ways to report:
- Report online at MedEffect
- By calling 1-866-234-2345 (toll-free);
- By completing a Consumer Side Effect Reporting Form and sending it by:
- Fax to 1-866-678-6789, or
- Mail to: Canada Vigilance Program
Health Canada
Postal Locator 1908C
Ottawa, Ontario K1A 0K9
Postage paid labels and the Consumer Side Effect Reporting Form are available at MedEffect.
NOTE: Contact your health professional if you need information about how to manage your side effects. The Canada Vigilance Program does not provide medical advice.
More information
Also, you can report any suspected adverse reactions associated with the use of health products to Merck Canada Inc. by one of the following 2 ways:
- Call toll-free at 1-800-567-2594
- Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-800-369-3090, or
- Mail to:
Merck Canada Inc.
Pharmacovigilance
16750 Transcanadienne
Kirkland, QC H9H 4M7
This document plus the full product monograph, prepared for health professionals can be found at http://www.alk.net or by contacting the sponsor, ALK-Abelló, Inc. at 1-800-325-7354 (for English) or at 1-800-663-0972 (for French).
Although the information shown in this document is current as of the date shown below, more current consumer information may be available at the manufacturer’s contact as given above.
This leaflet was prepared by ALK-Abelló A/S
Last revised: March 17, 2017
® Trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Used under license.
©2013, 2017 Merck Canada Inc., a subsidiary of Merck & Co., Inc., All rights reserved