Details for: SATIVEX
Company: GW PHARMA LIMITED
| DIN | DIN name | Active Ingredient(s) | Strength | Dosage Form | Route of Administration |
|---|---|---|---|---|---|
| 02266121 | SATIVEX | DELTA-9-TETRAHYDROCANNABINOL (CANNABIS SATIVA EXTRACT); CANNABIDIOL (CANNABIS SATIVA EXTRACT) | 2.7 MG / ACT; 2.5 MG / ACT | SPRAY | BUCCAL |
Consumer Information
This information was provided by the drug’s manufacturer when this drug product was approved for sale in Canada. It is designed for consumers and care givers. It is a summary of information about the drug and will not tell you everything about the drug. Contact your doctor or pharmacist if you have any questions about the drug.
What the medication is used for
SATIVEX® is used to relieve muscle stiffness in people with multiple sclerosis who do not get enough relief from other drugs they are using and who find additional relief with SATIVEX® .
What it does
SATIVEX® is used to improve symptoms related to muscle stiffness and make the muscles feel less stiff or rigid. You will know if your treatment is working if you have an improvement of your symptoms.
When it should not be used
- have a known or suspected allergy to any cannabis-based products, peppermintoil, propylene glycol or ethanol .
- have a serious heart problem such as angina, a previous heart attack, poorly controlled high blood pressure or a problem with your heart rate or heart beat.
- suffer from schizophrenia or depression.
- have a history of schizophrenia or any other psychotic disorder.
- are a child or adolescent under 18 years of age.
- are pregnant or nursing.
- are female at risk of pregnancy and not using a reliable contraceptive.
- are male and intending to start a family while on treatment with SATIVEX®
What the medicinal ingredient is
Cannabis sativa L. extracts Tetranabinex® and Nabidiolex® equivalent to 27 mg/mL delta-9-tetrahydrocannabinol (THC) and 25 mg/mL cannabidiol (CBD).
What the non-medicinal ingredients are
Ethanol, Peppermint oil (flavouring), Propylene glycol.
What dosage form it comes in
Buccal spray
27 mg/mL delta-9-tetrahydrocannabinol (THC) and 25 mg/mL cannabidiol (CBD).
Warnings and precautions
Serious Warnings and Precautions
- One of the ingredients in SATIVEX® can cause physical and psychological dependence and has the potential for being abused.
- SATIVEX® can cause changes in your heart. These include changes in your blood pressure, and a rapid heart beat. SATIVEX® is not recommended if you already have heart problems, including high blood pressure.
- Do not drive, operate machinery or carry out activities that require coordination and clear judgement.
- Talk to your doctor if you have a history of epilepsy or recurrent seizures.
To help avoid side effects and ensure proper use, talk to your healthcare professional before you take SATIVEX® . Talk about any health conditions or problems you may have, including if you:
- have epilepsy
- have dizziness or fainting
- have any kidney or liver problems
- are addicted to drugs or alcohol
- or anyone in your family has had schizophrenia or psychotic episodes
- are going to have surgery
- are taking other medicines.
Other warnings you should know about
- SATIVEX® may affect the way hormonal birth control methods, such as the “pill” or contraceptive implants, work. This means you should use and additional type of birth control. You and your partner must use a reliable barrier method of birth control, like a condom, diaphragm or cap. You should use these during your treatment with SATIVEX® and for at least 3 months after you stop taking SATIVEX® .
- If you see another doctor or go into hospital, let them know you are taking SATIVEX® .
- SATIVEX® contains about 50% v/v ethanol, which is an alcohol. Each spray contains about 0.04 g of alcohol. The usual daily dose will be greater than one spray. The amount of alcohol contained in SATIVEX® may be harmful if you have alcoholism. Your doctor will decide if you should still receive SATIVEX® if you have liver disease or epilepsy.
- SATIVEX® contains propylene glycol which may cause irritation.
Tell your healthcare professional about all the medicines you take, including any drugs, vitamins, minerals, natural supplements or alternative medicines.
Interactions with this medication
- sedatives/hypnotics like benzodiazepines, such as diazepam or triazolam; other sedatives, such as zopiclone, zolpidem, buspirone, St John’s Wort (a herbal preparation)
- muscle relaxants such as baclofen
- antibiotics such as rifampicin, clarithromycin
- epilepsy or nerve pain medications such as phenytoin, phenobarbital, carbamazepine
- statins to treat high cholesterol; such as atorvastatin or simvastatin
- antifungals such as itraconazole, fluconazole and ketoconazole
- corticosteroids used for inflammation such as hydrocortisone, beclomethasone, prednisolone
- some hormone medicines used for contraception or some types of cancer such as ethinyloestradiol, levonorgestrel or dydrogesterone
- anaesthesia to put you to sleep before an operation/surgery or for relaxing muscles before surgery, such as propofol
- drugs to treat HIV/AIDS such as ritonavir
- drugs to thin your blood, such as warfarin
- betablockers used to treat high blood pressure, such as bisoprolol, propranolol
- opioid pain relief products such as fentanyl, sufentanil and alfentanil
- antidepressants such as amitriptyline
- cannabis (marijuana, pot). Do not smoke marijuana while using SATIVEX® ,
- alcohol may interact with SATIVEX® , concentration and may affect your coordination and ability to respond quickly
- grapefruit juice
Proper use of this medication
- Follow the instructions in this section unless your doctor gives you different advice. If there is something you do not understand, ask your doctor or pharmacist. Continue to take this medicine for as long as your doctor prescribes.
- Spray SATIVEX® into your mouth, under your tongue or on the inside of your cheek.
- Do not spray the back of your throat. This will help you avoid inhaling SATIVEX® and will help you avoid getting an irritated throat.
- Spray SATIVEX® in different location in your mouth. This will help you to avoid stinging and discomfort in your mouth.
- Do not spray into your nose.
- There should be at least a 15 minute gap between sprays.
- If you experience any bothersome side effects, reduce your number of sprays or increase the time between each dose.
Usual Dose
You will determine the dose that is best for you. You can determine the dose that best suits you based on the relief you experience from taking SATIVEX® . You will figure out your regular daily dose by first starting slowly and increasing your dose gradually over the first few weeks of taking SATIVEX® .
- On day one, you should take one spray during the morning and one spray during the afternoon/evening. The morning dose can be taken at any time between waking up and 12 noon, and the afternoon/evening dose can be taken at any time between 4 pm and bedtime.
- After the first day, you can gradually and carefully increase your dose by one spray each day. Do this as you need it and based on how well you tolerate it until you experience improved relief of your muscle stiffness.
- When you have found a daily number of sprays that controls your muscle stiffness, you may adjust the timing between them, depending on how you feel.
- Keep your dosing schedule constant once you figure out the timing and number of sprays that best controls your muscle stiffness.
The average dose of SATIVEX® is 4 - 8 sprays per day. Most patients need 12 sprays a day or less. There is limited experience with doses higher than 12 sprays a day but you may need a higher number of sprays.
HOW TO USE YOUR SPRAY
When you first open a new vial:
- Shake the vial gently and remove the protective cap.
- Place the vial between your thumb and second finger with your first finger placed on the actuator.
- Press two or three times firmly and quickly into a tissue until a fine spray appears. See Diagram 1.
- You can now use SATIVEX®

SATIVEX® for daily use:
- Shake the vial gently before use.
- Remove the protective cap.
- Place the vial between your thumb and second finger with your first finger placed onthe actuator.
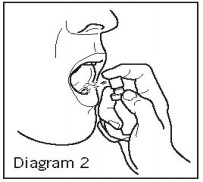
- Hold the vial in the upright position and direct the spray into your mouth under the tongue or onto the inside of the cheek. Hold your breath and press firmly and quickly. See Diagram 2.
- Replace the protective cap
Important:
- If you take 5 sprays each day you will notice after about 17 days that the noise of the spray action may change. You may also become aware of a different feeling in your mouth. This is indicating your medicine container is nearly empty. At this point start a new container of medicine.
- Keep spray away from eyes. If the spray comes into contact with your eyes or skin it should be washed away immediately with lots of water.
- Do not spray near children or pets.
- Do not use the spray near an open flame or heat source.
Overdose
If you think you have taken too much SATIVEX® , contact your healthcare professional, hospital emergency department or regional poison control centre immediately, even if there are no symptoms.
If you believe you have overdosed, you may have symptoms of intoxication, such as:
- hallucinations (seeing/hearing things that are not there)
- delusions (believing things that are not true)
- anxiety or paranoia (excessive anxiety or fear)
- increased or decreased heart rate with postural hypotension (feeling dizzy upon standing up).
If you need medical care, bring any remaining medicine and the container with you. Make a follow-up appointment with your usual doctor the day after an overdose
Missed Dose
If you forget to take a dose, do not worry. SATIVEX® is a medicine that is taken as required. Just take another as soon as you feel you need to.
Side effects and what to do about them
These are not all the possible side effects you may feel when taking SATIVEX® . If you experience any side effects not listed here, contact your healthcare professional.
You may have stinging or discomfort in your mouth if you spray SATIVEX® in the same place in your mouth on repeated occasions. You can help prevent this by varying the area in the mouth where you spray SATIVEX® . Do not continue spraying SATIVEX® onto sore or inflamed areas. Tell your doctor if your soreness persists.
If unacceptable and unwanted effects occur, stop taking SATIVEX® . These effects can be expected to wear off within a few hours. When returning to your medicine the dose should be reduced or the time between doses increased.
| Symptom / effect | Talk to your healthcare professional Only if severe | Talk to your healthcare professional In all cases | Stop taking drug and get immediate medical help |
|---|---|---|---|
| VERY COMMON | |||
| Fatigue | ✔ | ||
| Dizziness | ✔ | ||
| COMMON | |||
| Feeling over-excited or losing touch with reality | ✔ | ||
| Problems with your memory or having trouble concentrating | ✔ | ||
| Difficulty speaking | ✔ | ||
| Feeling disorientated or confused | ✔ | ||
| Depression (sad or low mood) | ✔ | ||
| Increase or decrease in appetite | ✔ | ||
| Feeling abnormal or drunk | ✔ | ||
| Changed sense of taste, or a dry mouth | ✔ | ||
| Loss of balance or falling over | ✔ | ||
| Nausea or vomiting | ✔ | ||
| Constipation or diarrhea | ✔ | ||
| Blurred vision | ✔ | ||
| Lack of energy or feeling weak or generally unwell. | ✔ | ||
| UNCOMMON | |||
| Seeing or hearing things that are not there (hallucinations) | ✔ | ||
| Thoughts about suicide | ✔ | ||
| Losing a sense of reality and not behaving normally | ✔ | ||
| Believing ideas that are not true | ✔ | ||
| Fainting | ✔ | ||
| Tooth or mouth discoloration | ✔ | ||
| Throat infection or irritation | ✔ | ||
| Cough | ✔ | ||
| Stomach pain | ✔ | ||
| Feeling people are against you or excessive fear and anxiety | ✔ | ||
| High or low blood pressure | ✔ | ||
| Rapid heartbeat | ✔ | ||
If you have a troublesome symptom or side effect that is not listed here or becomes bad enough to interfere with your daily activities, talk to your healthcare professional.
How to store
- Store upright.
- Store your unopened medicine in a refrigerator (2-8°C). Do not freeze.
- Once SATIVEX® is opened, use within 42 days. Opened vials of SATIVEX® may be stored at room temperature (15-25°C).
- This product is flammable. Do not leave SATIVEX in a hot place such as in direct sunlight or near a heat source.
- Do not use SATIVEX® after the expiry date shown on the product packaging. Return unused portion of SATIVEX® to the pharmacy for safe disposal or dispose of according to local regulations.
- Keep out of reach and sight of children.
- SATIVEX® may not be legal in other countries because it contains cannabis extracts. Before you travel with SATIVEX® , you may want to look up if cannabis containing drug products are legal in the country/region you will visit.
Reporting side effects
You can report any suspected side effects associated with the use of health products to Health Canada by:
- Visiting the Web page on Adverse Reaction Reporting (https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffectcanada/adverse-reaction-reporting.html) for information on how to report online, by mail or by fax; or
- Calling toll-free at 1-866-234-2345.
NOTE: Contact your health professional if you need information about how to manage your side effects. The Canada Vigilance Program does not provide medical advice.
More information
- Talk to your healthcare professional
- Find the full product monograph that is prepared for healthcare professionals and includes this Patient Medication Information by visiting the Health Canada website (https://health-products.canada.ca/dpd-bdpp/index-eng.jsp); the manufacturer’s website www.bayer.ca or by calling 1-800-265-7382
This leaflet was prepared by GW Pharma Ltd., Histon, Cambridge UK, CB24 9BZ
Last Revised December 11, 2019
Sativex® , Tetranabinex® and Nabidiolex® are registered trademarks of GW Pharma Ltd. used under licence by Bayer Inc. Bayer and the Bayer Cross are registered trademarks of Bayer AG, used under licence by Bayer Inc. 84472655