Details for: TUDORZA GENUAIR
Company: ASTRAZENECA CANADA INC
| DIN | DIN name | Active Ingredient(s) | Strength | Dosage Form | Route of Administration |
|---|---|---|---|---|---|
| 02409720 | TUDORZA GENUAIR | ACLIDINIUM BROMIDE | 400 MCG / ACT | POWDER (METERED DOSE) | INHALATION |
Summary Reports
Consumer Information
This information was provided by the drug’s manufacturer when this drug product was approved for sale in Canada. It is designed for consumers and care givers. It is a summary of information about the drug and will not tell you everything about the drug. Contact your doctor or pharmacist if you have any questions about the drug.
What the medication is used for
TUDORZA GENUAIR is used long term to help open the airways of people with breathing difficulties due to a lung disease called chronic obstructive pulmonary disease (COPD).
What it does
The active ingredient of TUDORZA GENUAIR is aclidinium bromide, which belongs to a group of medicines called bronchodilators. Bronchodilators relax airways and help keep bronchioles open. TUDORZA GENUAIR is a multidose dry powder inhaler that uses your breath to deliver the medicine directly into your lungs. This makes it easier for patients with COPD to breathe.
When it should not be used
Do not use TUDORZA GENUAIR if you:
- are allergic to aclidinium bromide or any of the other ingredients of this medicine.
- are under 18 years of age.
- experience sudden severe symptoms of COPD (called a COPD flare-up), such as sudden shortness of breath or wheezing. Your doctor may give you other medicine to use for sudden breathing problems as needed.
What the medicinal ingredient is
Aclidinium bromide
What the non-medicinal ingredients are
Lactose monohydrate.
What dosage form it comes in
400 mcg aclidinium bromide/inhalation
Warnings and precautions
BEFORE you use TUDORZA GENUAIR talk to your doctor or pharmacist if you:
- are pregnant or planning to become pregnant;
- are a breastfeeding mother;
- are taking any medications including eye drops, this includes medications you can buy without prescription;
- have had heart problems recently;
- have eye problems such as glaucoma, eye pain, blurred vision, see halos around lights or coloured images;
- have an enlarged prostate, problems passing urine, or painful urination;
- have a severe allergy to milk proteins. Ask your doctor if you are not sure;
- have had allergies to atropine or related medicines, for example ipratropium, tiotropium or oxitropium;
- have allergies to food or drugs.
TUDORZA GENUAIR should not be used more frequently than twice daily. Do not exceed the prescribed dose.
This medication has been prescribed for you and should not be given to other people.
Stop taking TUDORZA GENUAIR and seek medical help immediately:
- if you get tightness of the chest, coughing, wheezing or breathlessness immediately after using the medicine. These may be signs of a condition called bronchospasm.
Remember to tell any other doctor, dentist or pharmacist you consult that you are taking this medication.
Driving and using machines:
This medicine may cause headache and blurred vision. If you are affected by either of these side effects do not drive or use machinery until the headache has cleared and your vision has returned to normal.
Interactions with this medication
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Inform your doctor if you have been or are using similar medicines for breathing problems, e.g. medicines containing tiotropium, ipratropium or glycopyrronium. Ask your doctor or pharmacist if you are not sure. The use of TUDORZA GENUAIR with these medicines is not recommended.
Proper use of this medication
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
- The recommended dose is one inhalation twice a day, once in the morning and once in the evening.
- Use TUDORZA GENUAIR even when you have no breathing problems or other symptoms of COPD.
- Do not stop using the drug without consulting your doctor.
- You can use TUDORZA GENUAIR anytime before or after food or drink.
Usual dose
The recommended dose is one inhalation twice a day, once in the morning and once in the evening.
The recommended dose can be used for elderly patients and for patients with kidney or liver problems. No dose adjustments are necessary.
Overdose
If you think you may have used more TUDORZA GENUAIR than you should, contact your doctor or pharmacist.
Missed Dose
If you forget a dose of TUDORZA GENUAIR, inhale the dose as soon as you remember. However, if it is nearly time for your next dose, skip the missed dose.
Do not take a double dose to make up for a forgotten dose.
About your TUDORZA GENUAIR inhaler
Before using the TUDORZA GENUAIR inhaler, please read the full instructions. If you have any questions please ask your doctor or pharmacist.
Becoming familiar with TUDORZA GENUAIR: Remove the TUDORZA GENUAIR inhaler from the pouch and become familiar with its components.
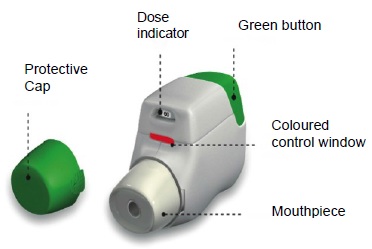
How to Use TUDORZA GENUAIR
Overview
To use your TUDORZA GENUAIR inhaler there are 2 steps you need to perform after removing the cap:
STEP 1: Press and RELEASE the green button and breathe out
completely, away from the inhaler.
STEP 2: Place your lips tightly around the mouthpiece and inhale STRONGLY and DEEPLY through the inhaler.
After inhalation, remember to replace the protective cap.
Getting Started
- Before first use, tear the sealed pouch along the notch and remove the TUDORZA GENUAIR inhaler.
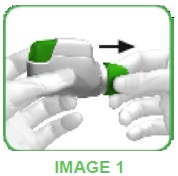
- When you are about to take your dose of medicine, remove the protective cap by lightly squeezing the arrows marked on each side and pulling outwards (see image 1).
- Look to see that nothing is blocking the mouthpiece.
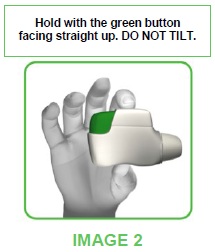
- Hold the TUDORZA GENUAIR inhaler horizontally with the mouthpiece towards you and the green button facing straight up (see image 2).
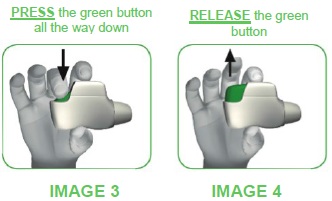
STEP 1: PRESS and RELEASE the green button and breathe out completely, away from the inhaler.
- Before bringing the inhaler to your mouth, press the green button all the way down (see image 3), then RELEASE it (see image 4).
DO NOT CONTINUE TO HOLD THE GREEN BUTTON DOWN.
Stop and Check: Make sure dose is ready for inhalation
- Make sure the coloured control window has changed to green (see image 5).
- The green control window confirms that your medicine is ready for inhalation.

IF THE COLOURED CONTROL WINDOW STAYS RED, PLEASE REPEAT PRESS AND RELEASE ACTIONS (SEE STEP 1).
- Before bringing the inhaler to your mouth, breathe out completely. Do not breathe out into the inhaler.
STEP 2: Inhale STRONGLY and DEEPLY through the mouthpiece.
- Put your lips tightly around the mouthpiece of the TUDORZA GENUAIR inhaler and breathe in STRONGLY and DEEPLY through your mouth (see image 6).
- This strong, deep breath pulls the medicine through the inhaler into your lungs.
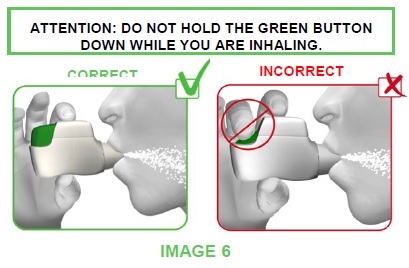
- While you breathe in you will hear a “CLICK” which signals that you are using the TUDORZA GENUAIR inhaler correctly.
- Keep breathing in even after you have heard the inhaler “CLICK” to be sure you get the full dose.
- Remove the TUDORZA GENUAIR inhaler from your mouth and hold your breath for as long as is comfortable, then breathe out slowly through your nose.
Note: Some patients may experience a mild sweet or slightly bitter taste, depending on the patient, when inhaling the medicine. Do not take an extra dose if you do not taste anything after inhaling.
Stop and Check: Make sure you have inhaled correctly
- Make sure the control window has turned to red (see image 7). This confirms that you have inhaled your full dose correctly.

IF THE COLOURED CONTROL WINDOW IS STILL GREEN, PLEASE REPEAT INHALING STRONGLY AND DEEPLY THROUGH THE MOUTHPIECE (SEE STEP 2).
- If the window still does not change to red, you may have forgotten to release the green button before inhaling or may not have inhaled correctly. If that happens, try again.
Make sure you have RELEASED the green button and take a STRONG deep breath in through the mouthpiece.
Note: If you are unable to inhale correctly after several attempts, consult your doctor.
- Once the window has turned red, replace the protective cap by pressing it back onto the mouthpiece (see image 8).

When should you get a new TUDORZA GENUAIR inhaler?
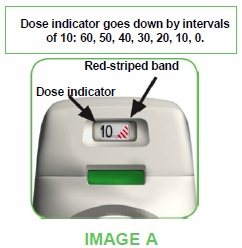
- The TUDORZA GENUAIR inhaler is equipped with a dose indicator to show you approximately how many doses are left in the inhaler. The dose indicator moves down slowly, displaying intervals of 10 (60, 50, 40, 30, 20, 10, 0) (see image A). Every TUDORZA GENUAIR inhaler will deliver the labeled number of doses.
When a red striped band appears in the dose indicator (see image A), this means you are nearing your last dose and you should obtain a new TUDORZA GENUAIR inhaler.
Note: If your TUDORZA GENUAIR inhaler appears to be damaged or if you lose the cap, your inhaler should be replaced. You DO NOT NEED to clean your TUDORZA GENUAIR inhaler. However, if you wish to clean it you should do so by wiping the outside of the mouthpiece with a dry tissue or paper towel.
NEVER use water to clean the TUDORZA GENUAIR inhaler, as this may damage your medicine.
How do you know that your TUDORZA GENUAIR inhaler is empty?
- When 0 (zero) appears in the middle of the dose indicator, you should continue using any doses remaining in the TUDORZA GENUAIR inhaler.
- When the last dose has been prepared for inhalation, the green button will not return to its full upper position, but will be locked in a middle position (see image B). Even though the green button is locked, your last dose may still be inhaled. After that, the TUDORZA GENUAIR inhaler cannot be used again and you should start using a new TUDORZA GENUAIR inhaler.

Side effects and what to do about them
Like all medicines, TUDORZA GENUAIR can cause side effects, although not everybody gets them.
Side effects may include:
- headache;
- nasopharyngitis (inflammation or irritation of the nose and throat);
- cough;
- falls and injury;
- sinus inflammation (sinusitis);
- blurred vision;
- hoarseness (dysphonia);
- dizziness;
- mouth or tooth infection;
- thrush in the mouth or throat;
- inflammation of the mouth (stomatitis);
- pain, stiffness and swelling in the joints;
- rash, skin itching.
| Symptom / effect | Talk with your doctor, nurse, or pharmacist only if severe | Talk with your doctor, nurse, or pharmacist in all cases | Stop taking drug and seek immediate medical help |
|---|---|---|---|
| Common | |||
| Nausea and/or diarrhea | ✔ | ||
| Uncommon | |||
| Dry mouth | ✔ | ||
| Heart failure: Fatigue; shortness of breath; or swelling of ankles or legs | ✔ | ||
| Hyperglycemia: High level of blood sugar (typical symptoms include excessive thirst or hunger and frequent urination) | ✔ | ||
| Vomiting and/or abdominal pain | ✔ | ||
| Faster heart beat | ✔ | ||
| Difficulty and pain passing urine or a feeling that your bladder has not completely emptied (urinary retention) | ✔ | ||
| Rare | |||
| Glaucoma: New or worsened pressure in your eyes, eye pain or discomfort, blurred vision, seeing halos of bright colours around lights, red eyes | ✔ | ||
| Unknown | |||
| Paradoxical Bronchospasm: Tightness of the chest associated with coughing, wheezing, or breathlessness immediately after inhalation of TUDORZA GENUAIR | ✔ | ||
| Serious allergic reactions: rash, hives, swelling of the face, mouth, lips and tongue with or without breathing problems | ✔ | ||
| Difficulty breathing | ✔ | ||
This is not a complete list of side effects. For any unexpected effects while taking TUDORZA GENUAIR contact your doctor or pharmacist.
How to store
Keep TUDORZA GENUAIR out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the inhaler label and carton after “EXP”. The expiry date refers to the last day of the month.
Store between 15 to 30°C.
Keep the TUDORZA GENUAIR inhaler protected inside the sealed pouch until you start to use it. Use the TUDORZA GENUAIR inhaler within 90 days of opening the pouch.
Do not use the TUDORZA GENUAIR if you notice that the pack is damaged or shows signs of tampering.
After you have taken the last dose, the inhaler has to be disposed of. You should follow local guidelines for domestic waste when throwing away the empty or unused inhaler. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
Reporting side effects
You can report any suspected adverse reactions associated with the use of health products to the Canada Vigilance Program by one of the following 3 ways:
- Report online at www.healthcanada.gc.ca/medeffect
- Call toll-free at 1-866-234-2345
- Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-866-678-6789, or
- Mail to: Canada Vigilance Program
Health Canada
Postal Locator 1908C
Ottawa, Ontario
K1A 0K9
Postage paid labels, Canada Vigilance Reporting Form and the adverse reaction reporting guidelines are available on the MedEffect™ Canada Web site at www.healthcanada.gc.ca/medeffect.
NOTE: Should you require information related to the management of side effects, contact your health professional. The Canada Vigilance Program does not provide medical advice.
More information
NOTE: This information for the Consumer Leaflet provides you with the most current information at the time of printing.
The most current information, the Consumer Information Leaflet plus the full Product Monograph, prepared for health professionals can be found at: www.astrazeneca.ca or by contacting the sponsor, AstraZeneca Canada Inc. at:
Customer Inquiries 1-800-668-6000.
This leaflet was prepared by:
AstraZeneca Canada Inc. Mississauga, Ontario L4Y 1M4
TUDORZA® and the logo are registered trademarks of Almirall, S.A., used under license by AstraZeneca Canada Inc.
GENUAIR® is a registered trademark of AstraZeneca UK Ltd., used under license by AstraZeneca Canada Inc.
The AstraZeneca logo is a registered trademark of AstraZeneca AB, used under license by AstraZeneca Canada Inc.
© AstraZeneca 2013-2017
Last revised: May 16, 2017