Details for: TOBI PODHALER
Company: BGP PHARMA ULC
| DIN | DIN name | Active Ingredient(s) | Strength | Dosage Form | Route of Administration |
|---|---|---|---|---|---|
| 02365154 | TOBI PODHALER | TOBRAMYCIN | 28 MG | CAPSULE | INHALATION |
Consumer Information
This information was provided by the drug’s manufacturer when this drug product was approved for sale in Canada. It is designed for consumers and care givers. It is a summary of information about the drug and will not tell you everything about the drug. Contact your doctor or pharmacist if you have any questions about the drug.
What the medication is used for
TOBI PODHALER is used to treat people (six years and older) with cystic fibrosis who
have a bacterial lung infection with Pseudomonas aeruginosa (see “What is
Pseudomonas aeruginosa?” section below).
Antibacterial drugs like TOBI PODHALER treat only bacterial infections. They do not
treat viral infections such as the common cold. Although you may feel better early in
treatment, TOBI PODHALER should be used exactly as directed. Misuse or overuse of
TOBI PODHALER could lead to the growth of bacteria that will not be killed by TOBI
PODHALER (resistance). This means that TOBI PODHALER may not work for you in
the future. Do not share your medicine.
What it does
TOBI PODHALER contains a medicine called tobramycin. Tobramycin belongs to the
aminoglycoside class of antibiotics. TOBI PODHALER is inhaled (breathe) directly into
the lungs so that the antibiotic can kill the Pseudomonas aeruginosa bacteria causing
the infection. This helps to fight lung infections and improve breathing in people with
cystic fibrosis.
What is Pseudomonas aeruginosa?
It is a very common bacterium that infects the lung of nearly everyone with cystic fibrosis
at some time during their lives. Some people do not get this infection until later on in
their lives, while others get it very young. It is one of the most damaging bacteria for
people with cystic fibrosis. If the infection is not properly fought, it will continue to
damage your lungs causing further problems to your breathing.
When it should not be used
Do not use TOBI PODHALER if you are allergic:
- to tobramycin, or to any other aminoglycoside antibiotic such as amikacin, gentamycin, kanamycin, paromomycin, streptomycin,
- to any of the other ingredients in TOBI PODHALER (see What are the ingredients in TOBI PODHALER?).
If this applies to you, tell your healthcare professional without taking TOBI PODHALER. If you think you may be allergic, ask your healthcare professional for advice.
What the medicinal ingredient is
Medicinal ingredients: Tobramycin
What the non-medicinal ingredients are
Non-medicinal ingredients: 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), blue ink, calcium chloride, carnauba wax, carrageenan, hypromellose, potassium chloride, sulfuric acid (for pH adjustment)
What dosage form it comes in
28 mg inhalation powder capsules
Warnings and precautions
To help avoid side effects and ensure proper use, talk to your healthcare professional before you take TOBI PODHALER. Talk about any health conditions or problems you may have, including if:
- You have hearing problems (including noises in the ears).
- You have vestibular problems (problems with your inner ear and brain) that can cause vertigo (loss of balance) and dizziness.
- You have kidney problems.
- You have unusual difficulty in breathing with wheezing or coughing and chest tightness.
- You have blood in your sputum (the substance you cough up).
- You have Parkinson’s disease.
- You have a condition called myasthenia gravis which is a chronic disease that causes muscle weakness.
- You are breastfeeding or planning to breastfeed.
- You are receiving an antibiotic called an aminoglycoside by injection since this can cause hearing loss, kidney problems or dizziness.
- You are taking any other medicines.
Other warnings you should know about:
Pregnancy:
Before taking TOBI PODHALER, talk to your healthcare professional if you are pregnant
or want to become pregnant. It is not known whether inhaling this medicine can harm an
unborn baby. When given by injection, the medicine in TOBI PODHALER can harm an
unborn baby and cause deafness. Your healthcare professional will talk to you about
whether you can take TOBI PODHALER if you are pregnant.
Breastfeeding:
When given by injection, the medicine in TOBI PODHALER can be found in the breast
milk. The quantity found in the breast milk after inhaling TOBI PODHALER is not known.
TOBI PODHALER may cause problems to your baby’s hearing or kidneys. Because of
the importance of the medicine to your well-being, you should stop breastfeeding or stop
taking TOBI PODHALER.
TOBI PODHALER is in a class of antibiotics that may cause hearing loss, dizziness, or
kidney problems. While you are using TOBI PODHALER and if you have or are at risk of
hearing or kidney problems, your healthcare professional may do bloodwork to check
how your kidneys are working. You may also take a hearing test to check whether or not
TOBI PODHALER is affecting your hearing.
Tell your healthcare professional about all the medicines you take, including any
drugs, vitamins, minerals, natural supplements or alternative medicines.
Interactions with this medication
You should not take the following medicines while you are taking TOBI PODHALER:
- Furosemide or ethacrynic acid, a diuretic (“water pills”)
- Urea or intravenous mannitol
You should not take the following medicines while you are taking TOBI PODHALER, or soon after finishing TOBI PODHALER treatment:
- Medicines (including tobramycin or another aminoglycoside antibiotic given by injection) that may harm your nervous system, kidneys or hearing. This interaction may cause hearing loss, dizziness, or kidney problems.
The following medicines can increase the chances of harmful effects occurring if they are given to you while you are receiving infusions of tobramycin or other aminoglycoside antibiotic. Talk with your healthcare professional before taking these medications:
- Amphotericin B, cefalotin, cyclosporine, tacrolimus, polymyxins: these medicines may harm your kidneys.
- Platinum compounds (such as carboplatin and cisplatin): these medicines may harm your kidneys or hearing.
- Anticholinesterases, (such as neostigmine and pyridostigmine), or botulinum toxin: these medicines may cause muscle weakness to appear or become worse.
Many other medications may also harm your nervous system, kidneys or hearing. Tell your healthcare professional about all the medications you are taking, even those that do not appear on this list.
Proper use of this medication
How to take TOBI PODHALER:
- TOBI PODHALER is a powder specifically formulated for inhalation using the PODHALER inhaler (see the step-by-step Instructions in the“How To Administer TOBI PODHALER” section below),
- Take TOBI PODHALER exactly as your healthcare professional tells you to. Ask your healthcare professional if you are not sure.
- Do not swallow the capsules.
- TOBI PODHALER capsules should be taken by inhalation using only the PODHALER that is provided in the pack.
- Each PODHALER is used for seven days and then discarded and replaced.
- No other capsules should be used with the PODHALER.
- Take TOBI PODHALER at the same time each day. This will help you remember when to take TOBI.
- Space the morning and evening doses as close as possible to 12 hours and not less than 6 hours apart.
- Please check the order of medications with your doctor. If you are taking several medications and have other therapies for cystic fibrosis, TOBI PODHALER should always be taken last. Take your medicines in the following order :
- 1 st bronchodilator
- 2 nd chest physiotherapy
- 3 rd other inhaled medications
- 4 th TOBI PODHALER
- Continue taking TOBI PODHALER as your healthcare professional tells you.
- If you have questions about how long to take TOBI PODHALER, talk to your doctor or your pharmacist.
Usual Dose
- Usual dose of TOBI PODHALER in adults and children 6 years of age and older:
- Inhale the content of 4 capsules (with 112 milligrams (mg) of tobramycin) in the morning and 4 capsules in the evening for 28 days using the PODHALER.
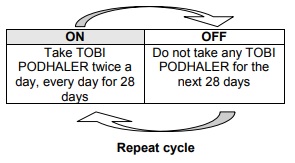
- After taking TOBI PODHALER for 28 days, stop using it and wait 28 days before starting another 28-days treatment cycle again.
- It is important that you keep using TOBI PODHALER two times per day during your 28 days on treatment and that you keep to the 28-day on, 28-day off cycle (see picture below).
Overdose
Missed Dose
- If you forget to take TOBI PODHALER and there are at least 6 hours to your next dose, take your dose as soon as you can. Otherwise, wait for your next dose.
- Do not take a double dose to make up for a missed dose.
Contents of the TOBI PODHALER Inhaler pack:
Each weekly box contains seven blister strips (corresponding to the seven days of the
week) and each blister strip contains eight capsules (corresponding to a daily dose:
content of 4 capsules to be inhaled in the morning and content of 4 capsules to be
inhaled in the evening). 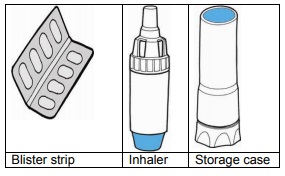
How to administer TOBI PODHALER:
This information is not intended to replace consultation with your healthcare professional,
and cystic fibrosis care team about properly taking medication or using inhalation
equipment.
| TOBI PODHALER Preparation | |
| 1. Wash and fully dry your hands. |  |
| Allow the device and capsules to reach room temperature before use. | |
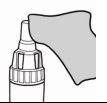
| 2. Just before use, remove the PODHALER from its case by holding the base and twisting off the top of the case in a counter-clockwise direction. |  |
| Set the top of the case aside. Briefly inspect the inhaler to make sure it is not damaged or dirty, then stand it in the base of the case. | |
| 3. Holding the body of the inhaler, unscrew and remove the mouthpiece from the inhaler body. Set the mouthpiece aside on a clean, dry surface. |  |
4. Separate the morning and evening doses from the blister strip as indicated in pictures
(1) and (2). Peel back the foil from the blister strip to reveal one TOBI PODHALER capsule and remove it from the card. 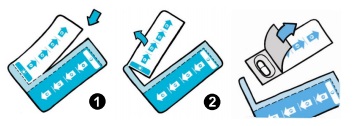 |
|
| Always keep the TOBI PODHALER capsules in the blister strip. Only remove a capsule just before you are going to use it. Do not store the capsules in the inhaler. | |
| 5. Immediately insert the capsule into the inhaler chamber (1). Never place a TOBI PODHALER capsule directly into the mouthpiece of the device. Replace the mouthpiece and screw it on firmly until it stops (2). Do not overtighten. |  |
| 6. To puncture capsule, hold the inhaler with the mouthpiece down, press the blue button firmly with your thumb as far as it will go, then release the button. Do not press the piercing button more than once at a time. The medication is now ready for inhalation. | 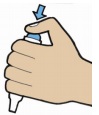 |
Occasionally, very small pieces of the capsule can get past the screen and get into your
mouth.
|
|
| TOBI PODHALER Inhalation | |
| 7. Fully exhale away from the inhaler. Never blow into the mouthpiece of the device. Position the inhaler with the mouthpiece facing towards you. Place mouth over the mouthpiece creating a tight seal. Inhale the powder deeply with a single continuous inhalation. Remove inhaler from mouth, and hold breath for a count of approximately 5 seconds, then exhale normally away from the inhaler. | 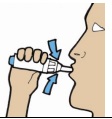 |
| 8. After a few normal breaths, perform a second inhalation from the same capsule, repeating step 7 above. | 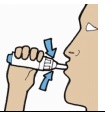 |
| Check and Continue | |
| 9. Unscrew mouthpiece (1) and remove the capsule from the chamber (2). | 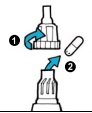 |
| 10. Inspect the used capsule. It should appear punctured and empty. If it is empty, discard the capsule. | 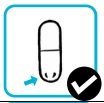 |
| If the capsule is punctured but still contains some powder, place it back into the chamber with the punctured side of the capsule inserted first, replace the mouthpiece and take another two inhalations from the capsule (repeat step 5, then steps 7 to 10 – do not repuncture the capsule). Reinspect capsule. | 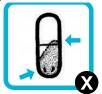 |
| If the capsule appears to be unpunctured, place it back into the chamber, replace the mouthpiece, press the button firmly as far as it goes and take another two inhalations from the capsule (repeat steps 5 to 10). After this if the capsule is still full and appears to be unpunctured, replace the inhaler with the reserve inhaler and try again (repeat steps 3, and 5 to 10). | 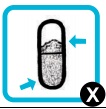 |
| 11. Repeat, starting at step 4 , for the remaining three capsules of the dose. | 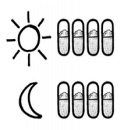 |
| 12. Replace the mouthpiece and screw it on firmly until it stops.
When the full dose (4 capsules) has been inhaled, wipe
mouthpiece with a clean dry cloth. The inhaler should never be washed with water. |
 |
| 13. Place inhaler back in storage case and close tightly. | 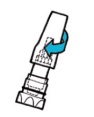 |
| Always keep the TOBI PODHALER capsules and device in a dry place | |
Older people
If you are aged 65 years and older, your healthcare professional may perform additional
tests to decide if TOBI PODHALER is right for you.
Children and adolescents
Caregivers should provide assistance to children starting TOBI PODHALER treatment,
particularly those aged 10 years or younger, and should continue to supervise them until
they are able to use the PODHALER device properly without help.
TOBI PODHALER can be taken by children and adolescents aged 6 years and older.
TOBI PODHALER should not be given to children less than 6 years old.
Driving and using machines
TOBI PODHALER should not affect your ability to drive and use machines.
Side effects and what to do about them
These are not all the possible side effects you may feel when taking TOBI PODHALER. If you experience any side effects not listed here, contact your healthcare professional. Some side effects are very common (these side effects may affect more than 1 in 10 patients):
- Cough.
- Difficulty speaking.
Some side effects are common (these side effects may affect between 1 and 10 in every 100 patients):
- Difficult or laboured breathing.
- Change in sense of taste.
- Mouth pain.
- Sore throat.
Some side effects are uncommon (these side effects may affect between 1 and 10 in every 1000 patients):
- Loss of voice (aphonia)
The frequency of some side effects is not known (the frequency cannot be estimated from the available data):
- Change in the colour of your sputum (substance you cough up).
- General feeling of being unwell.
If you experience symptoms such as severe diarrhea (bloody or watery) with or without
fever, abdominal pain, or tenderness, you may have Clostridium difficile colitis (bowel
inflammation). If this occurs, stop taking TOBI PODHALER and contact your healthcare
professional immediately.
Talk to your healthcare professional if the following occurs while taking TOBI
PODHALER
- If you are not getting better. Strains of Pseudomonas can become resistant to treatment with the antibiotic over time. This can mean TOBI PODHALER may not work as well over time.
| Symptom / effect | Talk with your doctor, nurse, or pharmacist only if severe | Talk with your doctor, nurse, or pharmacist in all cases | Stop taking drug and get immediate medical help |
|---|---|---|---|
| VERY COMMON | |||
| Worsening of your underlying lung disease | ✔ | ||
| Uncommon | |||
| Unusual difficulty in breathing with wheezing or coughing and chest tightness (bronchospasm) | ✔ | ||
| Coughing up blood | ✔ | ||
Hearing problems:
|
✔ | ||
| Shortness of breath, productive cough, sore throat, headache, fever | ✔ | ||
| Wheezing, rales (crackles), chest discomfort, chest pain from muscles and/or skeleton origins, decreased results for the tests of lung function, high level of sugar (glucose) in the blood | ✔ | ||
| NOT KNOWN | |||
Allergic reactions:
|
✔ | ||
If you have a troublesome symptom or side effect that is not listed here or becomes bad enough to interfere with your daily activities, talk to your healthcare professional.
How to store
- Store TOBI PODHALER capsules between 15-30°C.
- Store TOBI PODHALER capsules in the original package to protect from moisture.
- Store the inhaler in its tightly closed case when not in use.
- Do not use TOBI PODHALER beyond the expiration date stamped on the box.
- Keep out of reach and sight of children.
Reporting side effects
You can report any suspected side effects associated with the use of health products to Health Canada by:
- Visiting the Web page on Adverse Reaction Reporting (http://www.hc-sc.gc.ca/dhpmps/medeff/report-declaration/index-eng.php) for information on how to report online, by mail or by fax; or
- Calling toll-free at 1-866-234-2345.
NOTE: Contact your health professional if you need information about how to manage your side effects. The Canada Vigilance Program does not provide medical advice.
More information
If you want more information about TOBI PODHALER:
- Talk to your healthcare professional
- Find the full product monograph that is prepared for healthcare professionals and includes this Patient Medication Information by visiting the Health Canada website (http://hc-sc.gc.ca/index-eng.php); the manufacturer’s website www.mylan.ca, or by calling 1-844-596-9526.
This leaflet was prepared by
BGP Pharma ULC
85 Advance Road
Etobicoke, Ontario
M8Z 2S6
TOBI and PODHALER are registered trademarks of BGP Products Operations GmbH,
used under permission by BGP Pharma ULC, a Mylan company.
Last Revised July 22, 2019